Your cart is currently empty!
SwabCap® XT Luer Access Valve Disinfection Cap with 70% IPA
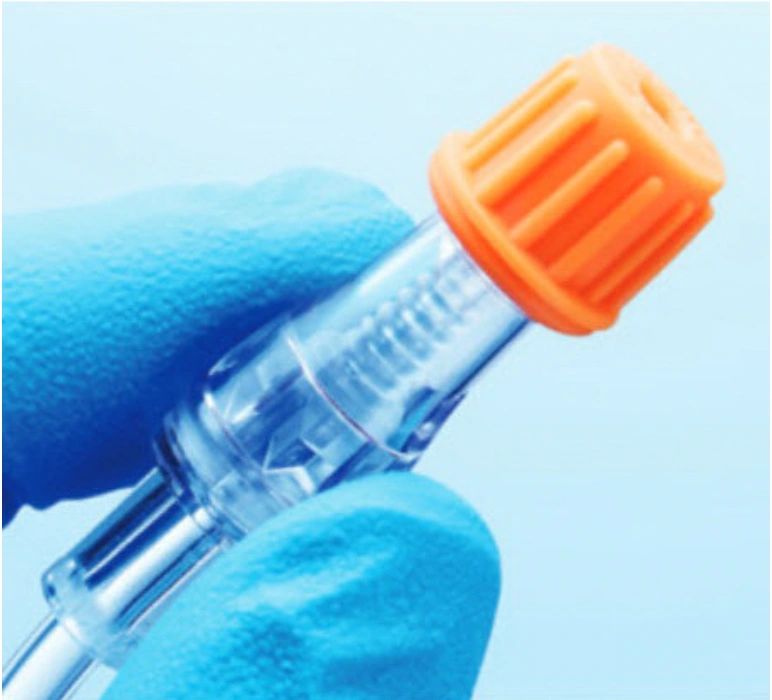
Manufacturer: ICU MEDICAL SwabCap® XT Luer Access Valve Disinfection Cap features: 70% IPA SwabCap will disinfect the valve 5 minutes after application and maintains a disinfected valve surface for up to 7 days if not removed Passive disinfection of valve top and threads without activating luer access valve Promotes technique standardization and compliance of luer access valve cleaning No swabbing
Description
Manufacturer: ICU MEDICAL SwabCap® XT Luer Access Valve Disinfection Cap features: 70% IPA SwabCap will disinfect the valve 5 minutes after application and maintains a disinfected valve surface for up to 7 days if not removed Passive disinfection of valve top and threads without activating luer access valve Promotes technique standardization and compliance of luer access valve cleaning No swabbing after removal Acts as a physical barrier from touch and airborne contamination Sterile, individually packaged Latex-free $149.99 BX of 200 EA | $1,499.90 CS of 10 BX California Proposition 65 Warning: ⚠ WARNING: These products can expose you to chemicals such as vinyl chloride, ethylene oxide, bisphenol-A (BPA), diisodecyl phthalate (DIDP), and/or di(2-ethylhexyl) phthalate (DEHP) which are known to the State of California to cause cancer and/or birth defects or other reproductive harm. For more information, go to www.P65Warnings.ca.gov. The above warning is required under California’s Safe Drinking Water and Toxic Enforcement Act of 1986, more commonly known as “Prop 65”. Some ICU products may be sterilized using ethylene oxide and some ICU intravenous tubing and drip chambers are manufactured using polyvinyl chloride (PVC) that may contain vinyl chloride, DIDP and/or DEHP. Also, certain manifold, stopcock, connector, and check valve products are manufactured using polycarbonates that may contain BPA. This shipment may contain one or more of these products.



Reviews
There are no reviews yet.